Perchloric Acid: A Detailed Commentary
Historical Development
Perchloric acid started to attract serious scientific curiosity during the early 19th century, when European chemists first uncovered its extreme reactivity and use as a potent oxidizer. Those early researchers didn’t have the modern equipment to handle it safely, and many learned the hard way about its dangers. Auguste-Jean-François Fourcroy is often linked to its initial studies, but it wasn’t until advancements in glassware and ventilation that the acid moved past laboratories and into broader industry. Over the decades, manufacturers refined the process to balance between output and safety. This push-and-pull between demand and disaster has marked the substance’s story. Each new incident forced changes in protocols and drove engineers to design more reliable containment and exhaust systems, laying groundwork for how we engage with hazardous materials today.
Product Overview
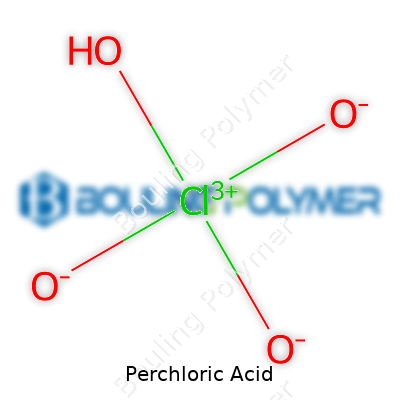
Perchloric acid, better known to chemists as HClO4, stands among the strongest mineral acids. In its pure form, you’ll find a colorless, oily liquid with fumes that call for respect. Laboratories keep it because nothing else can match its ability to clean tough organic residues or fuel rocket propellants. Folks dealing with analytical chemistry, electroplating, or etching head to perchloric acid for things sulfuric or nitric acids just can’t touch. Companies that process rare earth elements, metal ores, or heavy-duty lab glass swear by this reagent for digesting resistant samples.
Physical & Chemical Properties
This acid runs with a density around 1.768 g/cm3 at room temperature, and boils near 203°C. It dissolves easily in water, and that’s both a blessing and a hazard: dilute solutions act much milder than the pure stuff, which sits ready to trigger fires when mixed with organics. It doesn’t give you much warning—contact with combustible materials can turn a lab table into a chemical bonfire. Its high dielectric constant lets it cut through ionic bonds in ways few other acids can manage. Its hygroscopic tendencies mean it pulls moisture from the air, and storing it requires proper seals and regular checks.
Technical Specifications & Labeling
The packaging for perchloric acid reads like a checklist for survival. Anyone working with commercial concentrations, typically between 60% and 72%, will see hazard statements, corrosion pictograms, and specific instructions about using only glass or PTFE for transfer. Much laboratory supply comes with batch-specific purity certificates and warnings about volatile vapors. Regulated under GHS and various national guidelines, the acid falls into the highest risk categories for oxidizers and corrosives. Regulations force strict supply-chain documentation, and the inner containers often rely on pressure relief mechanisms to prevent dangerous build-up during storage or shipping mishaps.
Preparation Method
Factories don’t make perchloric acid lightly. Most commercial material comes from the reaction of sodium perchlorate and hydrochloric acid. Plant engineers drive this process in specialized reactors lined with Teflon or glass, separating the perchloric acid by distillation. The need to avoid metal contact at every step puts serious stress on equipment budgets and drives up costs. Some methods still use the older route—potassium perchlorate treated with concentrated sulfuric acid—but operators recognize the added sensitivity and waste streams that come with that choice. The pursuit of greener, lower-risk synthetic routes continues with every new wave of incidents.
Chemical Reactions & Modifications
Perchloric acid thunders through oxidation reactions, stripping electrons from organics and metals alike. Professionals working in analytical labs use it for digesting plant and biological samples—nothing else leaves as clean a residue. In synthesis, it creates perchlorate salts, important for explosives, propellants, and some specialized batteries. The acid catalyzes dehydration reactions and provides a key step in producing ammonium perchlorate, a must-have for solid rocket fuel. Any chemical engineer handling it will repeat the basics: separate perchloric from flammable solvents, never use with wood or cloth, and vent every workspace with robust wash-down hoods. Even a trace of organic matter can spell disaster in a hot, concentrated perchloric environment.
Synonyms & Product Names
Scientists and suppliers might call this material by many names—hydrogen perchlorate, hyperchloric acid, or sometimes just “PC acid.” Catalogs label it under UN1873 for transport, and folks working in explosives manufacturing nickname it “white devil” for its tendency to ignite like wildfire on slight provocation. Across languages, a quick glance at the structural formula sends a clear signal to treat with caution no matter what name appears on the bottle.
Safety & Operational Standards
The need for strong safety measures is not just for compliance but for survival. Labs that utilize perchloric acid never skimp on fume hoods. These aren’t basic chemical hoods; only ones with dedicated wash-down systems do the job, scrubbing away sticky perchlorate deposits before they build up to dangerous levels. Employees change gloves and lab coats more often than most, and every scrap of paper or cloth stays out of the working area. Industry guidelines—driven by agencies like OSHA and NIOSH—demand regular emergency drills, clear labeling, and strict chemical segregation. Storage comes down to using glass bottles, away from all combustibles and bases, with temperature logs and spill kits placed within arm’s reach. Even so, accidental mixes with reactive metals or certain plastics crop up and serve as tough reminders never to relax operational discipline.
Application Area
This acid earned its keep in more than just rocket science. Metallurgists rely on its unique etching power for sample prep and microstructure studies. Analysts dissect tough food or soil samples using perchloric acid digestion—a process that pulls out minerals or toxins for further testing. Chemical processors choose perchloric for making perchlorate salts, a core feedstock for flares, airbags, and fireworks. Electroplaters and those repairing scientific glassware use it for its unbeatable cleaning power, stripping away metal traces without scratching. Pharmaceutical research teams, though wary of toxicity, tap it for certain syntheses where only a non-nucleophilic acid will do. Each use comes with its own rulebook and respect for the substance’s destructive potential.
Research & Development
Push for better handling methods drives ongoing research. Engineers explore coatings and new materials to line containers, aiming to block acid migration and prevent disaster. Analytical chemists work to refine sensors for airborne perchlorates, giving earlier warnings of leaks or spills. Green chemistry specialists study alternative oxidizers in hopes of finding replacements that do the same job with less risk. Research centers team up with safety regulators to review incident data and test new exhaust filtration systems that snag vapors before reaching staff. This continuous drive, built on lessons from past accidents, points toward a future with fewer injuries and leaks.
Toxicity Research
Decades of animal and human studies show perchloric acid causes burns and tissue necrosis on contact. Even at lower concentrations, chronic inhalation leads to respiratory problems, and long-term skin exposure opens the door to deep ulcers. Research into environmental toxicity finds that perchlorates, the byproduct, persist in groundwater, moving faster than other ions and tainting crops. Experimental evidence from the CDC and EPA shows these ions block iodine uptake in the thyroid, stunting growth in children and weakening adult metabolism. Cleanup experts invent new filters to remove perchlorates from drinking water, but the process often runs slow and expensive. Anyone storing or using perchloric acid holds a responsibility to track and contain both vapor and liquid waste, facing fines and shutdowns for lapses.
Future Prospects
Growth in solid rocket propellants and modern electronics could drive up global demand, but environmental and health concerns keep that growth in check. Governments look closer at alternative cleaning agents and more sustainable oxidizers, hoping to shrink the chemical’s footprint in the next decade. Sustainable chemistry conferences flood with presentations on reducing perchloric acid waste or designing more efficient fume hoods. Adaptive manufacturing seeks ways to reclaim perchlorates from spent lab stock. In my years working with hazardous chemicals, I’ve watched safety standards push innovation across the board. For perchloric acid, the future holds the promise of smarter engineering—where necessity and caution keep pace with the demands of heavy industry and scientific discovery.
The Harsh Reality of Chemistry's Heavy Hitters
Perchloric acid doesn’t come up in everyday conversation. Yet, take a look behind the scenes in chemical labs, rocket test stands, or the factories making electronics—the place gets thick with it. This acid tears through stubborn roadblocks in chemistry. It’s a clear, colorless liquid, but the stuff demands respect. Spilled on clothes or the wrong countertop, it leaves nothing but regret and a bill for replacement tiles.
Why Scientists Reach for Perchloric Acid
Perchloric acid can tear apart complex substances in ways few chemicals manage. In analytical chemistry, and I’ve watched this process unfold, folks use it to digest samples. Soil, biological tissue, and food often need a hard reset before analysts can tease apart metals or other trace elements. Plenty of research labs still swear by perchloric acid for this stage—it rips apart organic matter, leaving a clear solution where later tests shine. The acid works fast, but labs need special fume hoods and gear. Safety is not negotiable.
Rocketry and the Power of Perchlorates
Flip over to rocketry, and the scale gets bigger. Perchloric acid helps create ammonium perchlorate, a main ingredient in solid rocket propellant. Think about NASA missions, fireworks, or even military uses. Ammonium perchlorate gives rockets a huge push. The chemical reaction throws a gut punch to gravity, lifting heavy payloads high into the sky. Every launch hinges on a chain of chemistry that starts with perchloric acid. In my work with science outreach, I’ve shown students how raw chemistry powers things like satellites or the big, bright shows on the Fourth of July. It’s not magic—it’s chemistry with teeth.
Electronics and Everyday Items
It surprises many people to learn that perchloric acid lands in the mix for making circuit boards. It etches metals cleanly and fast, making sure connections don’t break down later. Some companies use it to refine precious metals like gold and platinum. The acid pulls impurities away so the final product performs well in everything from phones to dental crowns. In school, I tried simple metal refining for a class project—using strong acids to separate out pure metals brought home how industrial chemistry shapes stuff we rely on daily.
Risks, Accidents, and How to Do Things Better
Perchloric acid doesn’t play nice in the wrong hands. If vapors build up around organic dust or old cloth, the risk of explosion creeps up. More than one fire department has dealt with lab explosions connected to improper handling of the acid. Working as a safety officer in a shared science building, I saw the cost of skipping steps with this chemical. Special hoods, spill plans, and real training keep things safe. Regulatory bodies and industry groups have pushed for better protocol—better training, clear storage, and no tolerance for shortcuts.
What the Future Holds
Clean chemistry matters more every day. The world leaps ahead in electronics, renewable energy, and research, but every step must keep people and the environment safe. Labs and factories get smarter by building in new safeguards and swapping out the most dangerous processes where possible. People in science have a responsibility to not just use powerful tools like perchloric acid, but truly respect them. Experience counts, and so does making sure the next generation of chemists and engineers know the risks—and the rules. The story of perchloric acid shows that progress walks hand-in-hand with safety, not in spite of it.
Perchloric Acid and Its Reputation
Perchloric acid often comes up in chemistry classes and industrial labs, yet most people have no idea how risky this chemical actually gets outside textbooks. I remember the anxiety in the lab the first time someone spilled a diluted sample—we cleared out fast, no questions asked. This stuff lives up to the warnings you hear in safety briefings.
What Makes Perchloric Acid So Dangerous?
Perchloric acid stands out in the world of strong acids. Its danger doesn't just come from its high acidity. The main issue stems from its strong oxidizing power. If you heat it up or concentrate it, you end up with a liquid that can trigger violent explosions. On top of that, it reacts with organic material or even certain metals and dust to create compounds known as perchlorates. Some of these perchlorates stay shock-sensitive and can detonate just from being scraped or jarred.
There’s a reason universities and research labs keep it locked up and reserved for very specific tests. In fact, many places install special “perchloric acid fume hoods” lined with stainless steel and water-wash systems. This isn’t overkill. Standard ducts or lab counters can build up perchlorate residues over time. A small spark, or even friction during routine cleaning, and you’re talking about a major fire—or worse.
Health Hazards: Not Just Burns
Many folks mistakenly link acids only to skin burns. With perchloric acid, skin burns are just one piece of the puzzle. Inhaling the fumes causes throat and lung irritation almost immediately. Prolonged exposure leads to serious lung damage. Eyes don’t fare much better; even short-term contact causes severe pain and can damage vision. There’s danger in contact, but also danger just from being nearby without the right equipment.
Chronic exposure poses risks, too. Studies have linked regular workplace use to anemia and problems with thyroid function, since perchlorate ions block iodine uptake in the body. Years back, lab techs whose only mistake was not using a good fume hood developed symptoms that stuck with them for life.
Why It Still Gets Used
Given all these risks, perchloric acid continues to turn up in laboratories and certain manufacturing processes. It’s especially important in making rocket fuel and explosives, and also in some food testing procedures, where nothing else gets the job done quite as well. Chemists sometimes call it “unforgiving” for good reason—it doesn’t allow shortcuts, ever. The payoff in performance comes at a high cost in safety protocols.
Staying Safe
Many organizations work hard to keep incidents from happening. The U.S. Environmental Protection Agency and OSHA both put out detailed guides on handling, labeling, and storing it. Training programs aren’t optional, and neither is special equipment. Anyone working with perchloric acid faces strict rules on lab layout, exhaust hoods, and protective gear.
Regular inspections and a strict no-food-or-drink policy work as basic principles in labs where perchloric acid gets used. I’ve seen colleagues double up on gloves and splash shields, refusing to cut corners even if it slows the work. If mistakes happen, quick access to extensive emergency eyewash stations and fountains saves injuries from getting worse.
Room for Improvement
Accidents involving perchloric acid sometimes start with forgotten details: an unnoticed residue on metal parts, or someone using the wrong kind of cleaning supplies. Good recordkeeping, better automation, and tireless education help, but nobody can afford to get careless. If you ask me, more regular drills and anonymous “close call” reporting could push things even further. Workers share tips and flag potential hazards before anything major happens, and that’s what keeps labs operating safely.
Not Your Average Lab Acid
Perchloric acid isn’t like vinegar or lemon juice—anyone who’s worked in a lab knows this. With enough strength to tear through organic matter and ignite materials on contact, perchloric acid changes the rules of chemical storage. I remember in my student lab days, even fresh graduates paid special attention to the yellow warning stickers on these bottles. The risk isn’t just from the acid itself; its fumes can turn innocuous dust or grease into explosive hazards. That stark warning sticks with people for a reason.
Location: Distance and Design Matter
The average chemical lockup cabinet won’t cut it here. Perchloric acid deserves a dedicated spot—far from any organic chemicals or reducing agents that might spark a violent reaction. Experts, including the National Research Council, agree that storing it in a segregated, well-ventilated acid cabinet (preferably stainless steel or ceramic, never wood or painted metal) lowers the risk. The room should be fitted with a special fume hood, the type that can handle corrosive vapors without letting perchlorate salts build up inside the ducts. Those salts can become untouchable explosives over time.
Humidity, Heat, and Direct Sunlight: Big No’s
High humidity gives perchloric acid more fuel for trouble. The more it absorbs water, the more it can corrode containers, turning shelves into hazard zones. Sitting the bottles away from direct sunlight or any source of heat avoids dangerous pressure buildup. Just thinking back to a spill in a warm storeroom—luckily it ended with a controlled clean-up, but the sharp, choking vapor left an impression.
Containers: Little Room for Error
Strong glass bottles or tightly sealed plastic containers (like PTFE or high-density polyethylene) keep acid from leaking or reacting with the container itself. No improvising with old lab glassware, since even a tiny scratch or a worn cap can spell trouble. Every label should stay sharp and clear, including hazard warnings. More than once, I’ve seen sloppy or faded labels in shared university storage closets, raising the chance for mistakes, especially during late-night experiments.
Everything Clean, Always
Perchloric acid reacts with organic residue that lingers in cabinets, shelf liners, or even dust. A routine wipe-down with water (never solvents or fuel-based cleaners) reduces the hidden risks. I’ve heard supervisors warn that a single paper towel, left damp and forgotten, could be trouble. Only store the acid in a spotless, clutter-free environment.
Training and Communication
Mistakes usually aren’t a result of malice; they happen when people don’t know, or forget, the rules. Frequent safety briefings, detailed signage, and color-coded storage layouts help keep everyone—newbies and veterans—on the same page. The important details stick when they’re part of daily habits, right down to the point of checking and double-checking before returning a bottle to its shelf.
Moving Past Complacency
The facts and stories around perchloric acid storage make a case that no shortcuts are worth the risk. Emergency showers, eyewash stations, and spill kits should always sit close by. Inventory logs prevent careless overstocking and forgotten half-finished bottles. Safety isn’t just about equipment; it’s about discipline, clean habits, and real respect for chemicals that carry consequences. People who store perchloric acid safely help everyone who shares that workspace make it home in one piece.
Concentrations You’ll Find on the Shelf
Ask any chemist about perchloric acid, and the first thing they mention is probably the sharp tang of caution in their voice. Perchloric acid isn’t your everyday chemical, and the concentrations matter a lot. Most suppliers offer solutions from about 60% up to 72%, since these strengths cover the usual needs in industrial, analytic, and academic labs. The 70% and 72% solutions pop up most often—these barely leave room for more water, which ramps up both the reactivity and risk involved.
Why Concentration Isn’t Just a Number
A number on a bottle doesn’t mean much until you try to work with it. Higher concentrations of perchloric acid don’t just mean stronger results; they bring more challenges. A 60% solution works for digesting samples and preparing certain reagents. Push past 70% and the acid gets fierce—reacting more violently with organic materials and metals. Industries using this acid for etching, battery electrolytes, or rocket propellants demand higher concentrations. So the stakes climb with each extra percent dissolved.
My Lab Experience: Respecting the Acid
The first time I made up a perchloric acid reaction, the protocol said “use a fume hood” in bold as if shouting. And for good reason. Even a small flask of 70% solution turns into a disaster if spilled on a wooden bench or rag. The vapors corrode, and drops can eat through skin and clothing. Working with lower concentrations, you sense you’re handling something strong. Jump above 70%, and you feel like you’re holding potential energy, ready to unleash. In one university lab, cleaner concentrations around 60% covered most of our analysis tasks. Nobody allowed anything close to the highly concentrated stuff near paper, wood, or any grease.
Risks and Real Talk About Safety
Perchloric acid sits on lists of dangerous substances for solid reasons. Above 72%, the acid forms azeotropes—mixtures with water that boil off together. Handling concentrated solutions, especially in warm labs, carries a real risk of explosion if organic material or metal shavings get involved. The Environmental Protection Agency and OSHA both put out strict guidelines; open containers only under strong ventilation, keep away from combustibles, and scrub every spill like you’re trying to erase history. When acids strong enough to oxidize most metals hit shelves, it’s not the time for shortcuts.
Solutions to Make Handling Safer
Choosing a lower concentration, if it fits the job, is a good move. Diluting the acid before it reaches the bench gives fewer headaches and cuts the danger if something goes wrong. On a bigger scale, labs that pull out perchloric acid regularly install special hoods with wash-down systems to remove hazardous buildup on ductwork. Good labeling, proper storage, and regular training for staff always help. Institutions can swap out perchloric acid for less hazardous acids if the reaction will tolerate substitution—which cuts down accidents over time.
Final Thoughts
In the end, that percentage on the bottle tells more than just strength; it signals the responsibility needed. Lab safety isn’t just a checklist—working with perchloric acid makes this fact hard to forget. Choosing the right concentration and handling it with respect keeps people safe and projects on track.
Understanding the Substance
Perchloric acid isn’t just any chemical sitting on a lab shelf. This clear, colorless liquid has serious power. With just a small concentration, it acts as a strong acid. In higher concentrations or heated conditions, it shows its explosive, oxidizing side. Most chemists respect it for cleaning lab glassware or preparing specialty chemicals, but respect without care turns dangerous quickly. I’ve worked in labs where the scent of danger comes not from the acid itself, but from how fast things can turn ugly. Burns, poison fumes, fire—perchloric acid can unleash it all with one mistake.
Personal Protective Gear: No Shortcuts
Anyone who’s spent time around perchloric acid knows gloves and goggles don’t cut it. Chemical splash goggles, a face shield, acid-resistant rubber gloves, and a full-length lab coat offer minimum protection. Shoes matter too—rubber or neoprene help protect feet from accidental spills, because this acid chews through fabric and skin faster than most realize. The one time a colleague forgot to button a lab coat, a few drops landed and left a scar as a reminder. Skip wearing contact lenses; fumes or splashes trap acids behind them.
Engineering Controls: Don’t Rely on Luck
Working with perchloric acid calls for a special fume hood—specifically designed so no vapors or condensed acids build up. Unlike regular hoods, these come with wash-down features to keep explosive perchlorates from forming in ductwork. I remember a story from grad school: a research facility ignored these precautions, and crystals inside a ventilation pipe led to an explosion during maintenance. No one expects fireworks from cleaning ducts, but that’s what they got. Never heat this acid outside a hood. Vapors can move, settle, and react days later. Keep a spill kit labeled for strong acids within arm’s reach.
Storage: More Than Just a Locked Cabinet
Store perchloric acid in a cool, well-ventilated area, away from organic materials including paper and wood. Anything flammable, like solvents or even dust, spells danger near this acid. Corrosive-resistant secondary containment makes leaks far less dramatic. Keep perchloric acid away from reducing agents, combustibles, and finely divided metals. The one time acids and organics mix, you can end up with an explosion, not just a ruined experiment.
Handling and Disposal: Always Thinking Ahead
Use only glassware free of organic residue for experiments involving perchloric acid. Even a fingerprint or leftover grease can trigger unexpected reactions. I once watched a small fire start from a drop hitting a bit of paper stuck to a clamp stand. Clean every surface, every tool, before bringing out this acid. Disposal isn’t straightforward; you can’t pour it down the drain or mix it with regular waste. Collect it in a dedicated, clearly marked container. Connect with hazardous waste professionals who know the rules—local codes treat this substance differently than weaker acids.
Training and Culture: Everyone on the Same Page
Training isn’t just a checkbox. New hires, seasoned researchers, visiting collaborators—everyone benefits from a serious walkthrough. It helps to schedule refresher classes. Real-world demonstrations of near-misses stay with people far longer than lectures ever will. Post clear instructions at handling sites. Even in a rush, the written rules keep everyone mindful. No shortcuts. I’ve seen teams grow lax over time, but it takes one surprise reaction to go from overconfident to careful.
Stronger Systems for Safer Labs
Lab safety starts with strong habits. Regular hazard assessments, well-maintained engineering controls, and a culture supporting protective gear matter every day. Encourage open conversations about mistakes and ways to improve. Stay ahead with frequent updates to training materials. Focusing attention on perchloric acid’s quirks protects people and property every step of the way.