Cyanuric Acid: More Than a Pool Chemical
Historical Development
Cyanuric acid’s journey started in the 19th century as chemists explored new compounds from simple cyanide salts. They paid close attention to the oddly stable ring it forms, shaping future applications. By the mid-20th century, research around pool chlorination put cyanuric acid into every household pool supply store. Scientists at the time worked with rudimentary glassware and trial-and-error synthesis, trying to make sense of nitrogen-rich compounds. They didn’t know the backyard swimming pool industry would grab their findings and run with it. Decades of water science, military applications, and chemical manufacturing have placed cyanuric acid in a wide array of uses few would have expected back then.
Product Overview
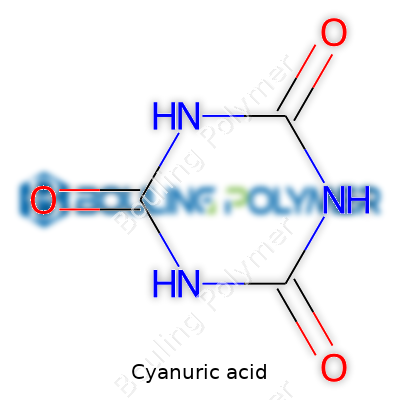
Cyanuric acid, known in the chemical world as 1,3,5-triazine-2,4,6-triol, usually takes the form of an odorless white powder or granule. Most people think of pool stabilizer, but chemical manufacturers see a raw material with a knack for holding onto chlorine, resisting sunlight. It shows up in water treatment, even crop protection. Large companies churn out metric tons each year for brands across continents. Behind every pail lie decades of process optimization, from safety checks to careful bagging that keeps out moisture. The big draw? Cyanuric acid extends the life of free chlorine in harsh sunlight, pushing down on chemical costs and letting users keep pools safer longer.
Physical & Chemical Properties
Cyanuric acid doesn’t win beauty contests: it sits dry, white, and somewhat chalky at room temperature. It holds a molecular weight of 129.07 g/mol and doesn’t dissolve much in cold water. Raise the heat, it grants a little more solubility, but nothing spectacular. Give it sulfuric acid or a strong base, and its stable triazine ring cracks apart into practical intermediates. The molecule packs three N-H bonds into a six-membered ring with alternating carbons and nitrogens—a design resistant to everyday wear and tear. It’s not volatile, won’t burn in your hand, and asks for respect only because of its dust, which can irritate lungs. Industry wants this sort of stability, especially in harsh or unpredictable environments.
Technical Specifications & Labeling
Down at the warehouse level, cyanuric acid gets slotted into a quality control grid. Purity tends to run 98% or higher for swimming pool and industrial use. Bags stand up to punctures, harsh storage, and moisture. Labels spell out hazard codes, batch numbers, recommended handling, and storage temperatures. Regulatory agencies in the US and Europe both demand that workers see warnings on inhalation risks and environmental disposal. Safety data sheets ride along in every shipment, sometimes translated into several languages for global trade. Concentrations drop into water at rates based on liters, gallons, or parts per million, depending on country and use. Mislabel a batch, and the risks escalate—not just for users, but for the people handling this product behind the scenes.
Preparation Method
Cyanuric acid doesn’t spring right from the earth. Most production today traces back to urea. Factories heat urea to high temperatures under controlled conditions, cycling ammonia and carbon dioxide as byproducts. The trimerization of cyanic acid, discovered through trial and adjustment, gives rise to that robust triazine ring. Refinement means washing out unreacted materials, drying under vacuum, and grinding to precise grain sizes. Each step introduces efficiency challenges, emission controls, and careful monitoring by chemists. Outdoors, the preparation happens on an industrial scale using dedicated reactors where temperature, reaction time, and incoming raw material quality set the pace for the final product. Smaller labs—often for specialty research—stick to older batch methods, but the outcome remains the same: white cyanuric acid, ready for duty.
Chemical Reactions & Modifications
Cyanuric acid has a stable ring, but in the hands of experienced chemists, it plays along with a handful of reactions. Under the right conditions, it reacts with chlorine or sodium hypochlorite to give trichloroisocyanuric acid and dichloroisocyanuric acid, both heavyweights in water disinfection. Acid catalysts or tough oxidation break the ring—yielding carbon dioxide, ammonia, and cyanic acid, among other fragments. Researchers hunt for new derivatizations, hoping to squeeze out new flame retardants, herbicides, and specialty resins. The molecule’s symmetry and nitrogen load make it a starting point for custom triazine chemistry. Every new modification writes another chapter in the chemical playbook for applications stretching from plastics to complex pharmaceuticals.
Synonyms & Product Names
Product catalogs often refer to cyanuric acid by names like isocyanuric acid, tricarbimide, and 2,4,6-trihydroxy-1,3,5-triazine. The pool world calls it conditioner, stabilizer, or pool chlorine protector, while agrochemical suppliers list it as an intermediate without much fanfare. Other synonyms pop up in regulatory lists and shipping manifests across borders, sometimes in hard-to-pronounce forms. These alternative names matter when tracking imports, regulatory filings, or ensuring buyers get the right compound for the job.
Safety & Operational Standards
Handling cyanuric acid inside a plant draws on decades of lessons about containment and inhalation hazards. Dust can cause lung irritation; industry workers don protective masks and gloves. Factories invest in dust collection, employee rotating shifts, and eyewash stations nearby. Regulators set occupational exposure limits. Transporters carry the compound in sealed bags or drums, keeping out of rain and sparks. Waste protocols guide everything from rinsing empty bags to neutralizing spills. The environmental side grabs plenty of headlines—cyanuric acid breaks down in soils at a snail’s pace, so disposal needs a plan. Standards from OSHA, EPA, and ECHA guide producers and users, but daily diligence by workers and managers keeps people and waterways out of trouble.
Application Area
Most folks meet cyanuric acid next to a pool—its main claim to fame. It stops free chlorine from vanishing in the sun, slashing chemical bills and improving swimmer safety by keeping out bacteria, algae, and other nasties. Commercial pools measure cyanuric acid down to the part-per-million. Agribusiness taps it for slow-release fertilizers and herbicide manufacturing, piggybacking on its conversion into other triazines. Some industrial users blend it into plastics and resins to create fire-resistant materials. Military groups have studied its breakdown under extreme conditions for water provisioning. Even drinking water plants in drought-prone regions have weighed the pros and cons of using cyanuric acid–based disinfectants to keep supplies safe. Clearly, its impact reaches much farther than the nearest lawn chair.
Research & Development
Laboratories never really stand still. Ongoing work looks at safer forms, smarter dosing, and biodegradable alternatives. Universities explore how cyanuric acid interacts with common waterborne pollutants, searching for ways to neutralize threats. The focus stretches to reducing chemical footprints in recreational waters, as overuse can complicate pool chemistry and strain water systems. Teams in Asia and North America have published studies on using advanced oxidation or microbial digestion to break down leftover cyanuric acid after pool draining. Scientists at agricultural research stations keep testing new triazine derivatives for more selective weed-killing or improved crop nutrition. The race is always on for a greener, safer triazine world.
Toxicity Research
Toxicologists have dug deep. Short-term animal studies and human exposure tracking point to cyanuric acid as low in toxicity, at least in the amounts found in swimming pools or environmental samples. Acute ingestion causes mild gastrointestinal upset. Chronic overexposure runs the risk of damaging kidney function—this has shown up in rare cases where water treatment accidents led to much higher than normal concentrations. Environmental researchers have raised questions about impacts on fish and aquatic invertebrates, especially where pools get drained into storm drains. Regulatory agencies keep a wide safety margin, nudging pool owners and local governments to stick close to guidelines and enforce regular water testing.
Future Prospects
Looking ahead, cyanuric acid faces both opportunity and skepticism. The pool and spa market won’t disappear—rising temperatures and urbanization mean more cities are thinking about public water recreation. Agriculture might lean into new triazine developments for fertilizers and crop protection as climate stress tightens food supplies. Regulatory conversations build over newer, safer alternatives, likely spurring shifts toward molecules that break down faster in the environment. More efficient manufacturing tech could cut energy demands, emissions, and waste. As more consumers press for transparency and safety, cyanuric acid’s labeling, stewardship, and life-cycle impacts will draw a sharper focus. Any company banking on this compound should weave flexibility into their R&D, keeping at arm’s reach both steady profit and rapid adaptation when science or regulators tug the rug in a new direction. Anyone with skin in the cyanuric acid market will need eyes on both global pool trends and stricter standards for water chemistry and environmental safety.
Keeping Chlorine Working in the Sun
Sunshine feels great on the skin, but it’s rough on pool chemistry. Everyone who’s owned a backyard pool learns this the hard way. You toss in chlorine, and then—poof—it vanishes after a day in strong sunlight. Without help, you can end up pouring in more chlorine than water just to keep things sanitary. Here comes cyanuric acid, also called CYA. It works as a sunscreen for chlorine. Instead of letting UV rays chew up the chlorine, CYA helps it stick around, letting you keep the water clean without breaking the bank or your back.
How Cyanuric Acid Protects Your Pool
Most pool owners run into two big issues: dirty water or burning through chlorine too fast. Studies from the Centers for Disease Control show that sunlight can destroy over half the chlorine in a pool within two hours when there’s no cyanuric acid. With CYA mixed in, the chlorine lingers up to eight times longer. This means less time fiddling with chemicals and more time swimming.
Cyanuric acid isn’t some fringe trick. Just about every outdoor pool—public or private—relies on it. Health officials even recommend certain levels for safety. Without it, public pools would need to close down every afternoon just to reload on sanitizer.
Too Much of a Good Thing
I’ve seen new pool owners get excited and dump in way more CYA than they need, thinking more protection must be better. Too much, and chlorine stops fighting germs like it should. Recent research shows that high levels of cyanuric acid slow down chlorine’s attack on dangerous bugs, even the nasty ones that cause diarrhea outbreaks. Health experts say to stay under 50 parts per million (ppm), but I wouldn’t go above 30 or 40. Test kits aren’t just for show—use them.
In older pools, years of adding “stabilized” chlorine tablets (which pack in cyanuric acid) can push levels sky high. If you see green water or can’t shake that musty smell, it might not be a chlorine problem at all—it could be CYA overload. Partial draining and refilling can help, but no miracle chemical fixes this fast.
Simple Rules for Safer Swimming
Taking care of pool water shouldn’t feel like rocket science. Pick up a decent test kit. Check your cyanuric acid level at least once every couple months, more often in hot weather. If things get out of hand, remember that draining some pool water and refilling with fresh water works. Don’t trust chlorine tablets and “shock” packets alone. Many of these sneak in more cyanuric acid without warning.
At the end of the day, a balanced pool keeps friends safe and water clear. Cyanuric acid saves a ton of chlorine during summer, but regular checks stop small maintenance habits from turning into big problems. Knowing what goes in the pool protects everyone—including the person paying for all those chemicals.
Reliable Sources and Real Experience
I learned these hard lessons maintaining pools for family and neighbors, but public health experts back it up. Agencies like the CDC and EPA point straight to regulated, measured cyanuric acid use. Mixing a bit of science with simple observation keeps summer fun and safe. Trust testing over wishful thinking, and clear water will follow.
What Is Cyanuric Acid?
Cyanuric acid usually makes headlines in the world of swimming pools. Sold as a white, powdery chemical, this stabilizer keeps chlorine from breaking down in sunlight. Pool owners often rely on it, especially in hot, sunny climates. Folks who swim a lot, plus pets who love to splash around, sometimes wonder: Am I in the clear with this stuff lingering in the pool?
How Much Is in the Water?
Cyanuric acid levels in pool water matter more than most think. The Centers for Disease Control and Prevention recommends keeping pool cyanuric acid under 100 parts per million (ppm), but many pool manuals suggest between 30 and 50 ppm for best performance and safety. Too much, and chlorine takes a hit; water loses its punch against germs. In my hometown, our community pool once spiked above 150 ppm because staff tried to “protect the chlorine” during a hot spell. Swimmers started getting itchy skin and red eyes—not fun.
What Happens to People and Pets?
The real risk comes in gulping down water with cyanuric acid, not just touching it. The Environmental Protection Agency classifies it as having low toxicity by skin or lung exposure. Swallowing tiny amounts happens now and then, especially with kids. Nausea could follow if big gulps go down, but there’s no record of lasting harm at standard swimming levels. Dogs and cats who lap up pool water now and then aren’t likely to see problems, but drinking from the pool every day can add up, especially if chemicals are off-balance. My neighbor’s golden retriever once got sick after a pool party—not from one drink, but from constantly guzzling chlorinated, stabilized water that week.
Digging into Scientific Guidance
Regulatory agencies like the EPA and World Health Organization set guidance values to keep accidental exposures safe. One animal study found that rats handled drinking cyanuric acid at levels far higher than any pool, without health trouble, over weeks. No studies in humans have shown direct links between cyanuric acid and cancer or genetic harm. Still, no one should treat it as safe for sipping—standards for drinking water run much tighter than swimming pools.
How to Keep Pools Safe for All
Careful pool care makes the biggest difference. Pool test kits sold at most hardware stores check cyanuric acid with a color-change dot. Checking the water weekly, then draining a fraction and adding fresh water each season, keeps levels in check. If a pool tastes bitter or stings the eyes, it usually means the chemical load—chlorine, cyanuric acid, or both—has climbed too high. For homes with young children or pets, making water bowls available away from the pool can keep accidental gulps at bay. After heavy rains, topping off with hose water before adding more chemicals keeps everyone happier and less exposed.
The Bottom Line
Cyanuric acid has a job to do in the pool, guarding chlorine so it kills germs longer. Kept in the recommended range and managed with care, it won’t cause issues for swimmers or four-legged pool fans. Drinking pool water never counts as a healthy habit, whether the threat is chlorine, salt, or stabilizers. With regular testing and common sense, both people and pets can stick to swimming, not sipping.
Why Cyanuric Acid Deserves Your Attention
Cyanuric acid sits on most pool supply shelves, but a lot of pool owners pass it by without much thought. I once ignored it too, but a few hot summers and a couple of green pools later, I realized this chemical shapes whether chlorine works to keep water clear. Sunlight eats chlorine up faster than most people expect. Cyanuric acid works as protection. If you live in a sunny area or use your pool often, missing the right level creates cloudy water, irritation, and extra expenses. In my experience, checking this level turns an okay pool into trouble-free fun.
How to Test Cyanuric Acid Levels—Simple Steps for Good Results
Most test kits make the process simple. A good test kit includes a vial, a labeled reagent, and clear instructions. Fill the sample vial with pool water taken from at least elbow-deep in the pool—not right next to a jet or step. Add the reagent. The water turns cloudy. Pour the solution into the provided viewing tube until you can just barely see the black dot at the bottom. Compare the fill line to the markings, which show the cyanuric acid level in parts per million (ppm). Some strips offer a shortcut and display an approximate range after dipping them in water, but most pool professionals and seasoned owners trust liquid test kits for readings.
Putting off this test leads to wasted money on chlorine, or a pool that needs constant cleaning and shock treatments. After battling algae in July, I started testing every other week and noticed fewer issues immediately. Most guides and experts point to 30–50 ppm as a good target for backyard pools. Numbers above 80 ppm slow chlorine down so much that it stops fighting off the germs and algae.
Why Getting Reliable Numbers Calls for a Little Effort
The reliability of your test hinges on a clean vial and fresh reagent. Old test kits collect dust and sunlight in garages, causing unpredictable results. Buying new reagents at the start of each swim season pays off. If the results keep surprising you, compare your home tests with a free check at a pool store. I did this early on and spotted a big gap from my out-of-date kit, which led to my pool turning green just before a family cookout.
Many questions about water problems come back to unbalanced cyanuric acid. Chlorine tablets often sneak in more of this chemical. Without watching the level, you risk letting it creep too high over time. Draining and refilling a portion of water solves this, but regular checks prevent the hassle.
Solving Pool Headaches Before They Start
Testing for cyanuric acid isn’t glamorous, but it saves both time and headaches. Skipping it can turn quick fixes into all-day filter cleans or emergency store runs. Professional recommendations stress record-keeping and consistency. Jot the results down each time. Catching a problem early saves money, water, and prevents missing a pool day now and then. For any pool owner who enjoys hassle-free swimming, taking a few minutes to test cyanuric acid pays off every summer.
Why Pool Owners Keep an Eye on Cyanuric Acid
I’ve known plenty of pool owners who keep a closer watch on their chlorine levels than their bank accounts. Yet, it’s Cyanuric Acid (CYA) that often tells the real story for anyone hoping to swim in clear, safe water. This chemical, often called “pool stabilizer,” serves as sunscreen for your chlorine. Without it, sunlight eats up free chlorine at a surprising rate, forcing people to pour in more and more chemicals. Getting the level right means you’re not just saving money, you’re creating a pool that’s comfortable for swimmers and safe from bacteria or algae.
Finding the Right Balance: The Sweet Spot
Through years of talking with pool professionals and reading up on various pool chemistry guides, I’ve seen a clear consensus: the ideal range for cyanuric acid sits right between 30 and 50 parts per million (ppm) for most outdoor pools. Levels within this range offer solid UV protection for chlorine without tying up the sanitizer or compromising water quality. If the level drops below 20 ppm, UV rays make quick work of chlorine and owners soon watch their sanitizer vanish. On the other hand, letting CYA rise above 60 ppm doesn’t just protect chlorine—it cages it up, making the sanitizer sluggish and less effective at fighting bacteria or algae blooms.
Health and Enjoyment Matter Too
High CYA can creep up if folks keep shocking their pools with stabilized chlorine or tablets, unaware they’re adding both sanitizer and more stabilizer each dose. I remember one summer when a friend’s pool turned cloudy, and tests showed CYA over 100 ppm. The chlorine couldn’t keep up, algae flourished, and the only fix was draining part of the pool—costly, wasteful, and time-consuming. Swimmers want to hop into water that looks good and feels good. Highly stabilized water with too much CYA can lead to eye irritation and a lingering chemical scent as owners overcompensate with more chlorine, chasing clean water that never quite arrives.
Best Practices for Pool Owners
Testing matters. I once relied on a pool store for advice but found that home test kits offered quicker and more frequent feedback. Most reliable kits use a simple white dot or turbidity test for CYA concentration. Weekly checks during summer, especially in pools using stabilized chlorine, can save headaches later in the season. If levels climb too high, partial draining and refilling offer the surest solution—no chemical can “remove” excess CYA, despite what some products claim.
Unstabilized chlorine, such as liquid bleach or cal-hypo tablets, helps maintain chlorine without bumping up CYA. Some pool owners swap these oxidizers into their routine to avoid rising stabilizer. Regular backwashes (for sand or DE filters) and topping off with fresh water help bring down CYA. Embracing these habits leaves you with more control and fewer surprises.
Trustworthy Resources Lead to Better Outcomes
Every pool owner faces a learning curve. Turning to well-regarded sources—the Centers for Disease Control and Prevention recommends 30-50 ppm CYA for effective chlorine—helps keep your water clear and inviting. Learning from experience, connecting with trusted professionals, and staying up-to-date with pool chemistry facts pays off both in fewer chemical costs and countless enjoyed summer days.
Understanding Why Cyanuric Acid Matters
You might not give cyanuric acid much thought until pool water starts turning cloudy or chlorine just won't last more than a day. I’ve been there, watching my backyard swim spot swing between sparkling and sad depending on what the test strips show. Cyanuric acid, or stabilizer, acts like sunscreen for the chlorine in your pool, slowing down how fast the sun eats it up. Too little, and your sanitizer burns off before it can do its job. Too much, and you wind up with a pool where chlorine can’t handle the bacteria and algae.
Most pool owners shoot for a range of 30 to 50 ppm for cyanuric acid. Saltwater pools often drift a little higher. My experience lines up with what health departments recommend — balance is key. Too little, and you’re always dumping in chlorine. Too much, and pool chemistry gets stubborn.
What Goes Wrong — The Highs and Lows
In spring, I tested my pool and got hit with a number well over 100 ppm. The water looked fine, but chlorine kept disappearing and algae started creeping in around the steps. Industry research shows high cyanuric acid locks up chlorine, turning your sanitizer into a couch potato that can’t keep the pool healthy. On the flip side, if cyanuric acid drops too low, sunlight burns away chlorine so quickly you’d think someone turned on a UV laser above the water.
Every pool owner I know faces this at some point. Rain, backwashing, and splash-out lower cyanuric acid. Tablet chlorine, many shock products, and some winterizing chemicals raise it over time. Nobody wants to buy endless test kits just to keep things in check, but that short weekly check-in saves a ton of hassle.
Bringing Levels Down — What Actually Works
The only way I’ve knocked down high cyanuric acid is with plain water replacement. No magic chemical pulls it out, no quick fix from the pool store despite their best sales pitch. Drain off some water, fill up with fresh, and then retest. I’ve seen people chip away at high levels by doing partial drains of 25% at a time, especially if water restrictions make a full drain impossible or irresponsible. That big hassle is why it pays to keep a close eye on stabilizer before it climbs out of reach.
A few companies promote CYA reducers, but lab and field testing from research publications rarely backs up strong claims for these products. If you go that route, keep your expectations in check and test to see what actually changes in your pool.
Raising Cyanuric Acid Without Overdoing It
If test results show stabilizer dipping below 30 ppm and the sun chews through chlorine by mid-afternoon, time to add cyanuric acid. Powdered stabilizer works fine — dissolve it slowly, using a sock or skimmer basket, then give it time to spread. If you choose chlorine tablets, remember those add stabilizer along with sanitizer, so watch your CYA rise over the season. Too many pool owners wind up with high stabilizer after a summer of using only tablets.
Smart Pool Chemistry Means Fewer Headaches
Nobody wants to spend backyard time chasing chemical numbers. Upkeep with cyanuric acid keeps the pool inviting and cuts down on cost and effort. Testing every week, logging numbers on a phone app or notepad, and nudging the chemistry before it snowballs makes pool ownership feel a lot more relaxed. It’s a small thing that goes a long way, and after fixing a cloudy mess one summer, I never skip my stabilizer check again.